NIST SRM 1401 NIST SRM 1401 冷凍人類血液中的追溯金屬
NIST SRM 1401
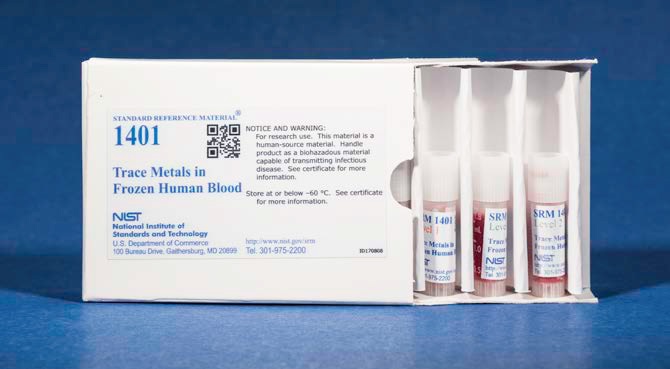
NIST SRM 1401 冷凍人類血液中的追溯金屬
NIST SRM 1401 Trace Metals in Frozen Human Blood
廠牌:代理
供應商:大台北尖端儀器有限公司
聯絡電話:02-2531-0981
| 快速詢價 |
幾十年來,基於金屬 - 金屬機械結構的植入裝置已廣泛用於人體關節手術復位。隨著時間的推移,這些接合系統可能會磨損或降解,釋放潛在有毒金屬如鈷和鉻的顆粒進入血流。對患者健康的影響是十分重要的,因此臨床實驗室準確測量全血中極低濃度的金屬離子對於設備故障預測和患者健康管理非常重要。這些類型的測量是具有挑戰性的,因為外來污染,儀器靈敏度和乾擾很容易導致數據中的測量偏差。一般來說,這些測量類型的實驗室性能相對較差。為了解決這些問題,國際管理機構正在製定行動標準並製定正在通過能力驗證計劃進行評估的績效標準。 NIST已與疾病控制和預防中心合作,為評估與能力驗證活動相關的績效標準以及方法開發和驗證提供關鍵質量保證支持的新標準參考材料(SRM)。 SRM 1401冷凍人血液中的追溯金屬由四個冷凍人血液瓶,兩個不同濃度水平的兩個小瓶組成。每個級別都通過鉻,鈷,錳和鉬的質量分數認證。 SRM旨在補充聯合王國LGC正在開發的類似參考資料。
Implant devices based on metal-on-metal mechanical structures have been extensively used in human joint surgical re-habilitation for several decades. Over time, these joint systems can wear or degrade, releasing particles of potentially toxic metals such as cobalt and chromium into the blood stream. The impact on patient health is of substantial concern, and the accurate measurement of metal ions in whole blood at very low concentration levels by clinical laboratories is therefore extremely important for device failure prediction and patient health management. These types of measurements are challenging because exogenous contamination, instrument sensitivity, and interferences can easily cause measurement biases in the data. In general, laboratory performance for these types of measurements has been relatively poor. To address these issues, international regulatory agencies are establishing action levels and developing performance standards that are being assessed through proficiency testing schemes. NIST has partnered with the Centers for Disease Control and Prevention to produce a new Standard Reference Material (SRM) providing critical quality assurance support for assessing performance standards related to proficiency testing activities, as well as method development and validation. SRM 1401 Trace Metals in Frozen Human Blood consists of four vials of frozen human blood, two vials each of two different concentration levels. Each level is certified for mass fractions of chromium, cobalt, manganese, and molybdenum. The SRM has been designed to complement a similar reference material being developed by LGC in the United Kingdom.